Description
Clonality: Monoclonal
Host: Mouse
Purification: Supernatant
Reactivity: Human, Bovine
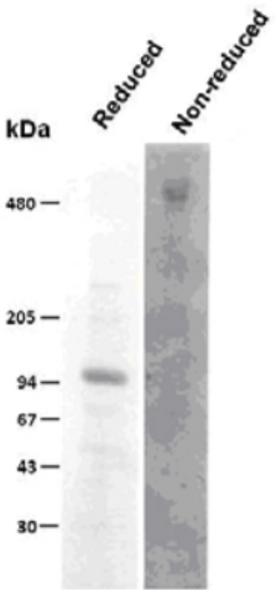
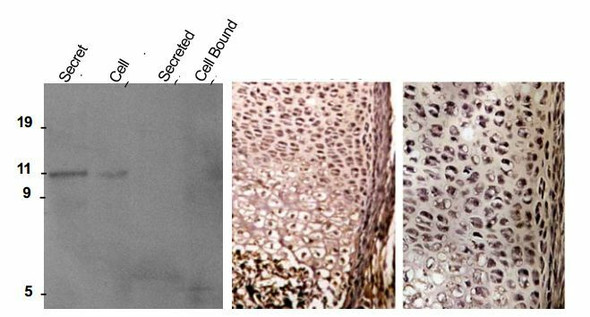
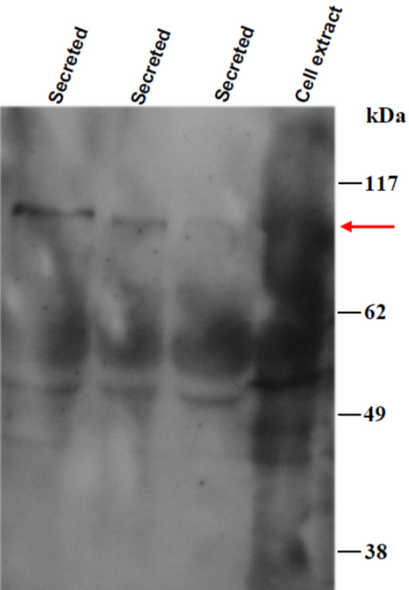
Biglycan is a small secreted ECM proteoglycan belonging to the small leucine-rich repeat (SLRP) subfamily. It contains a central 12 LRR domain flanked by small cysteine clusters at either side. The structure of biglycan core protein is highly conserved across species; over 90% has been reported for rat, mouse, bovine and human biglycan core proteins. Two glycosaminoglycan chains of the chondroitin or dermatan sulfate type are attached near the amino terminus of the core protein, generating a molecule that in its fully glycanated form reaches 250 kDa. Deposition of non-glycanated forms of biglycan have been shown to increase in cartilage and bone ECMs with age. Like decorin and fibromodulin, biglycan controls collagen fibrillogenesis and, partly through this action, it is believed to play a key role in bone mineralization and the assembly of cartilage and corneal ECM. In fact, deletion of the biglycan gene leads to an osteoporosis-like phenotype and double knockout of biglycan and fibromodulin cause severe cartilage and macular degeneration. The biglycan core protein binds BMP4 and may influence its bioactivity, especially in the context of osteoblast differentiation/maturation. This is believed to depend upon the ability of biglycan to regulate the interaction of the growth factor with its extracellular antagonist chordin. There is also evidence that biglycan may bind TGFb1 and affect homeostasis and new formation of blood vessels. Furthermore, BGN may affect signal transduction during cell growth and differentiation via induction of the cyclin-dependent kinase inhibitor p27KIP1. Biglycan-induced activation of RhoA and Rac1 signaling increases migration of lung fibroblasts. Moreover, adenovirus-mediated gene transfer of BGN induced a fibroblastic response in the lung, indicating a role of BGN in fibrogenesis. Finally, biglycan is up-regulated during inflammation and augments this condition by contributing to Toll-like receptors 2 and 4 signaling in macrophages.