COX-3, a cyclooxygenase-1 variant inhibited
Acetaminophen is often classified as a non-steroidal anti-inflammatory drug (NSAID), although in clinical practice and in animal models it has little anti-inflammatory activity (1). However, like NSAIDs, acetaminophen inhibits pain and fever and is one of the most popular pain relievers / fever reducers in the world. Despite the long-term use and popularity of acetaminophen, it lacks a clear mechanism of action. Flower and Vane demonstrated that acetaminophen inhibited cyclooxygenase (COX) activity in dog brain homogenates more than in spleen homogenates (2). This gave rise to the concept that there are variants of COX enzymes that are differentially sensitive to this drug and that acetaminophen acts centrally. However, although two isozymes of COX are known, neither isozyme is sensitive to acetaminophen at therapeutic concentrations of the drug in whole cells or homogenates. In contrast, COX-1 and -2 in homogenates frequently exhibit the paradoxical property of being stimulated by sub-millimolar concentrations of acetaminophen and inhibited by super-millimolar levels of the drug (1). This finding suggests that no isozyme is a good candidate for the site of action of acetaminophen.
When analyzing the expression of COX-1 and -2 RNA in dog tissues, our laboratory observed that the cerebral cortex of the dog brain contains two different RNAs that hybridized with a canine COX-1 cDNA. One RNA was ~ 2.6 kb in size and the other was ~ 1.9 kb in size, and analyzes of these RNAs suggest that they encode previously uncharacterized COX-1 related proteins.
Materials and methods
Unless otherwise noted, all basic protocols used were from the Sambrook and Russell manual on molecular cloning (3).
RNA isolation and construction of a cDNA library.
RNA isolation and library construction methods have been described (4). Human Multiple Tissue Northern Blots (MTN) were purchased from CLONTECH.
Antisense oligonucleotides for the first intron of human and canine COX-1 genes were synthesized and end-labeled using [γ-32P] dATP.
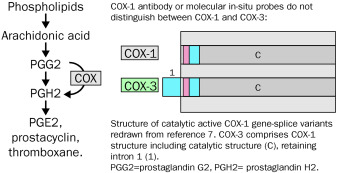
A canine cerebral cortex cDNA library was screened using a ~ 1.0 kb canine COX-1 fragment previously cloned in this laboratory by reverse transcription (RT) coupled PCR. The library was also screened with a 32P-labeled canine COX-1 intron 1 antisense oligonucleotide. Two full-length clones were isolated, fully sequenced, and named COX-3 and partial COX-1a (PCOX-1a). Both were derived from the canine COX-1 gene but retain intron 1. PCOX-1a also has a 657 bp in-frame deletion spanning exons 5-8.
RT-PCR of canine and human cerebral cortex mRNA.
CDNA was synthesized from the canine cerebral cortex and primers were designed for PCR amplification. The sense primer (5′-CGGATCCGCCGCCCAGAGCTATGAG-3 ′) corresponded to nucleotides 15-32 of the canine COX-3 sequence (sent to GenBank under accession number AF535138), with the 3 ′ end of the primer two nucleotides downstream of the initiator. methionine. The antisense primer (5′-cgccatcctggtgggggtcaggcacacgga-3 ′) corresponded to nucleotides 1865-1894, located 32 nucleotides upstream of the stop codon.
Northern blot analysis of human tissues with an intron probe 1 detected a 5.2 kb mRNA similar in size to one previously reported (5). The marathon-ready human cerebral cortex cDNA (CLONTECH) was amplified by PCR (CLONTECH, PCR Advantage 2 Enzyme System), using 5 'and 3' primers, and an amplified 4.2 kb fragment was recovered and found to contain the entire coding region of human COX-1 with retained intron 1.
Expression of COX-3 and PCOX-1a in Baculovirus.
Both COX-3 and PCOX-1a were cloned into the baculovirus expression vector pBlueBac 4.5 / V5-His (Invitrogen). Sf9 cells (~ 1 × 106) were infected with viral stocks with a multiplicity of infection (moi) of 3 for the expression of COX-3, PCOX-1a, mouse COX-1 and mouse COX-2 (6).
In some cases, tunicamycin at a final concentration of 10 µg / ml was added to the insect cells 1 hr after infection, and the cells were cultured and harvested after 48 hr. The activity of intact cells was determined by RIA (7).
Detection of 60, 53 and 50 kDa COX-1 related proteins in human aortic tissue.
Total protein (20 µg) from human aorta was analyzed by Western blot, using COX-1 mAb (Cayman Chemical, Ann Arbor, MI) and COX-3 antipeptide polyclonal antibodies (pAb). Primary antibodies were preincubated with a mixture of human and mouse COX-1 intron 1 peptide (described below) for 1 hr at 4 ° C, or left unblocked. The