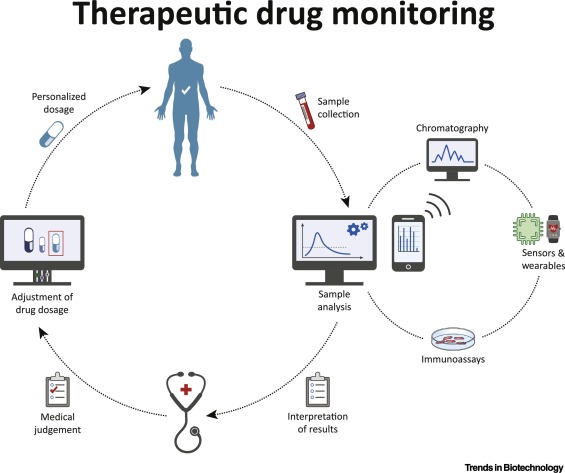
On-Site Therapeutic Drug Monitoring
Highlights
On-site therapeutic drug monitoring has the potential to improve patient outcomes and drastically reduce healthcare costs.
Despite being on the radar of the scientific community for almost two decades, sensor-based approaches have yet to break through and support the clinical application of therapeutic drug monitoring, potentially due to the gap between scientific and clinical communities.
Chromatography as a routine practice is limited due to its lack of standardization, high turnaround-times and instrumentation costs, and labored sample preparation.
Sensors offer a low-cost, easy-to-use, and on-site analysis method to explore the full potential of therapeutic drug monitoring, overcoming these limitations.
The success of individualized dosing strongly relies on two factors: how PK / PD studies are integrated with therapeutic drug monitoring and how the measurement process is managed.
Recent technological advances have stimulated efforts to bring personalized medicine into practice. Yet, traditional application fields like therapeutic drug monitoring (TDM) have remained rather under-appreciated.
Owing to clear dose-response relationships, TDM could improve patient outcomes and reduce healthcare costs. While chromatography-based routine practices are restricted due to high costs and turnaround times, biosensors overcome these limitations by offering on-site analysis.
Nevertheless, sensor-based approaches have yet to break through for clinical TDM applications, due to the gap between scientific and clinical communities. We provide a critical overview of current TDM practices, followed by a TDM guideline to establish a common ground across disciplines. Finally, we discuss how the translation of sensor systems for TDM can be facilitated, by highlighting the challenges and opportunities.