Description
Application: IHC
Clonality: Monoclonal
Conjugation: Biotin
Host: Mouse
Purification: Purified - Affinity
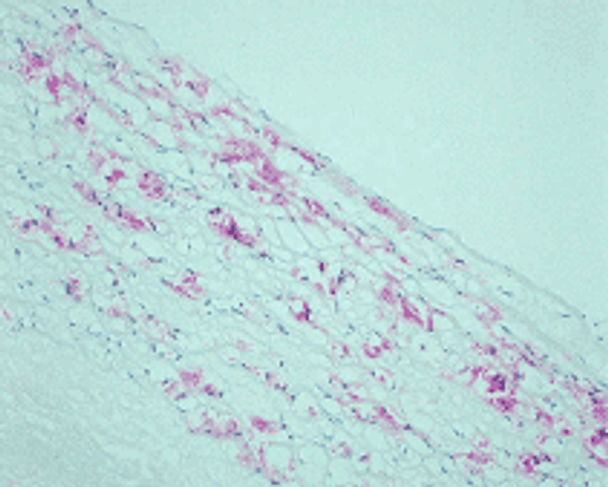
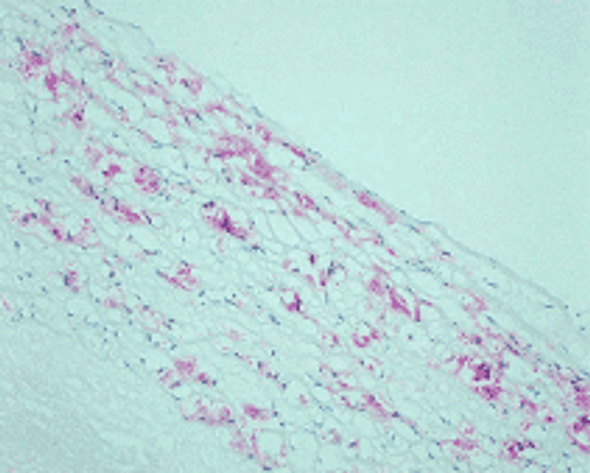
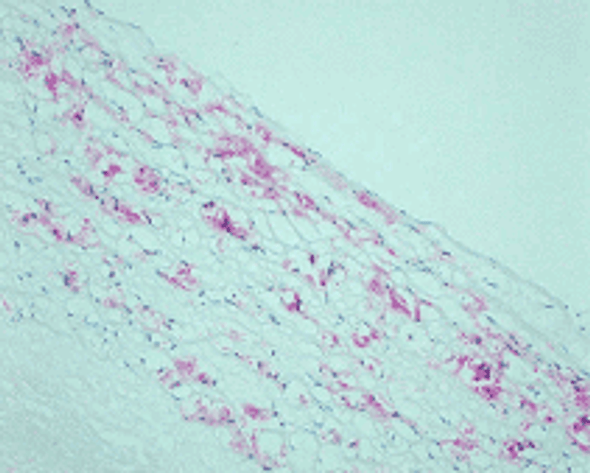
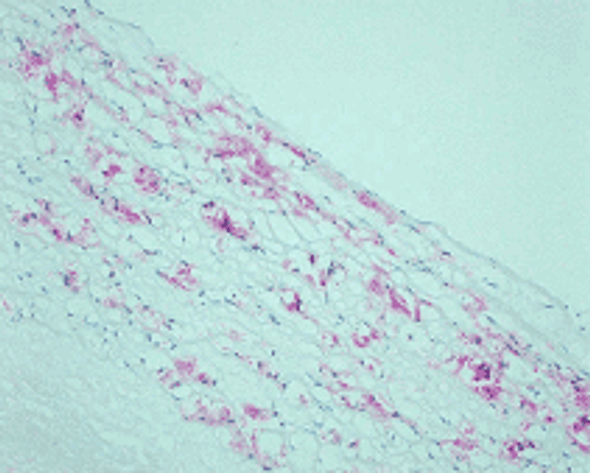
Reaction of protein amino groups with glucose leads, through the early products such as a Schiff base and Amadori rearrangement products, to the formation of advanced glycation end products (AGEs). Recent immunological studies using anti-AGEs antibody (6D12) demonstrated the presence of AGEs-modified proteins in several human tissues: (i) human lens (nondiabetic and noncataractous), (ii) renal proximal tubules in patients with diabetic nephropathy and chronic renal failure, (iii)diabetic retina, (iv) peripheral nerves of diabetic neuropathy, (v) atherosclerotic lesions of arterial walls, (vi) 2-microglobulin forming amyloid fibrils in patients with hemodialysis-related amyloidosis, (vii) senile plaques of patients with Alzheimer's disease, (viii)the peritoneum of CAPD patients, (ix) skin elastin in actinic elastosis, and (x) ceriod/lipofuscin deposits. These results suggest a potential role of AGEs-modification in normal aging as well as age-enhanced disease processes.This antibody named as 6D12 has been used to demonstrate AGEs-modified proteins in these human tissues, indicating potential usefulness of this antibody for histochemical identification and biochemical quantification of AGEs-modified proteins.
N'-(carboxymethyl)lysine (CML) is a major antigenic AGEs structure in vivo and is known to be generated from Oxidative cleavage of Amadori product. In addition to amadori product, CML formation also takes place through glyoxal, which is generated from theautoxidation of glucose and unsaturated fatty acids. NF-1G is monoclonal antibody specific for CML and useful for immunohistochemical staining to demonstrate the localization of CML in some pathological tissues.
View AllClose
Clonality: Monoclonal
Conjugation: Biotin
Host: Mouse
Purification: Purified - Affinity
Reaction of protein amino groups with glucose leads, through the early products such as a Schiff base and Amadori rearrangement products, to the formation of advanced glycation end products (AGEs). Recent immunological studies using anti-AGEs antibody (6D12) demonstrated the presence of AGEs-modified proteins in several human tissues: (i) human lens (nondiabetic and noncataractous), (ii) renal proximal tubules in patients with diabetic nephropathy and chronic renal failure, (iii)diabetic retina, (iv) peripheral nerves of diabetic neuropathy, (v) atherosclerotic lesions of arterial walls, (vi) 2-microglobulin forming amyloid fibrils in patients with hemodialysis-related amyloidosis, (vii) senile plaques of patients with Alzheimer's disease, (viii)the peritoneum of CAPD patients, (ix) skin elastin in actinic elastosis, and (x) ceriod/lipofuscin deposits. These results suggest a potential role of AGEs-modification in normal aging as well as age-enhanced disease processes.This antibody named as 6D12 has been used to demonstrate AGEs-modified proteins in these human tissues, indicating potential usefulness of this antibody for histochemical identification and biochemical quantification of AGEs-modified proteins.
N'-(carboxymethyl)lysine (CML) is a major antigenic AGEs structure in vivo and is known to be generated from Oxidative cleavage of Amadori product. In addition to amadori product, CML formation also takes place through glyoxal, which is generated from theautoxidation of glucose and unsaturated fatty acids. NF-1G is monoclonal antibody specific for CML and useful for immunohistochemical staining to demonstrate the localization of CML in some pathological tissues.